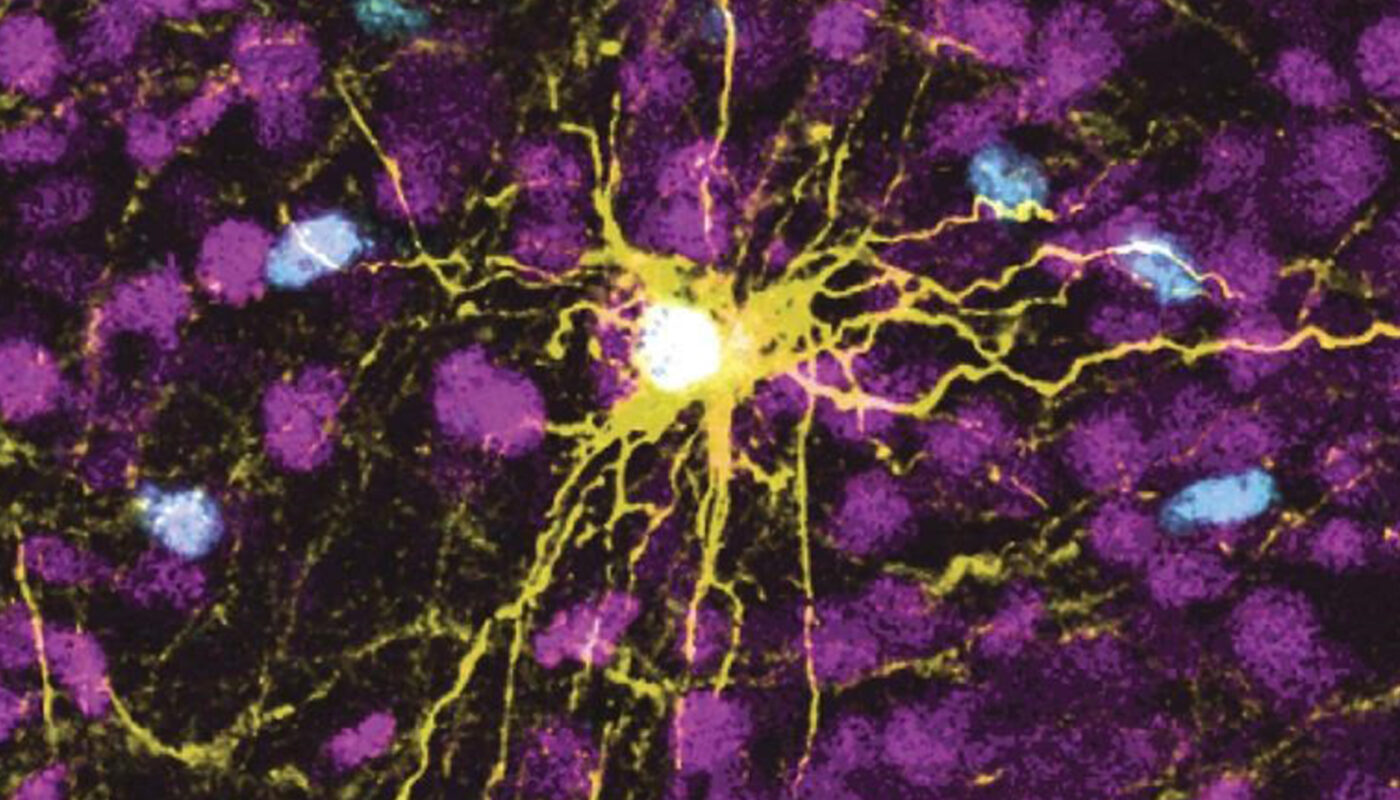
Researchers from the University at Buffalo have made a breakthrough in understanding the mechanisms behind infantile cystinosis, a rare disease that severely affects patients’ lifespan by causing kidney failure. The study, published in the International Journal of Molecular Sciences, demonstrates that the disease could potentially be cured through the use of the genome-editing technique CRISPR. This breakthrough discovery could potentially eliminate the need for kidney transplants, which is currently the most effective treatment for patients with infantile cystinosis.
Infantile cystinosis is the most severe and common form of cystinosis, a condition characterized by the accumulation of cystine, an amino acid, in the body’s cells. This build-up damages cells throughout the body, particularly in the kidneys and eyes. Current treatment options involve medications to lower cystine levels and therapies to address the impaired growth caused by the disease. In severe cases, patients may require feeding tubes and eventually dialysis and kidney transplants.
The researchers used human-induced pluripotent stem cells (hiPSCs) to study the genetic basis of this disease. HiPSCs have the ability to differentiate into various cell types, making them a valuable tool for studying genetic diseases. However, differentiating hiPSCs into kidney cells has proven challenging. The research team developed a protocol that successfully differentiated hiPSCs into kidney proximal tubules, the specific nephron segment affected by infantile cystinosis.
The protocol involved extracting stem cells from both a healthy individual and an individual with infantile cystinosis. The researchers developed a culture medium containing specific components found in blood, enabling the successful differentiation of hiPSCs into kidney cells. This breakthrough allowed the researchers to express markers important for the kidney’s reabsorptive functions, mimicking the disease’s effects on the proximal tubules.
This discovery opens the door for the potential use of the CRISPR genome-editing technique to repair the defective genome and cure the disease. By introducing the normal gene into the cystinotic hiPSCs, it is possible to replace the defective proximal tubules in patients with infantile cystinosis. Since these tubules are derived from the patient’s own cells, there should be no issues with tissue rejection.
The implications of this study extend beyond infantile cystinosis, as other kidney diseases involving damage to the proximal tubules could potentially benefit from this research. Acute kidney injury, which can lead to chronic kidney disease and renal failure, is one such example. The researchers acknowledge that further studies are necessary, including animal models and in vitro tissue culture cells, to validate these findings.
This groundbreaking research provides hope for patients with infantile cystinosis and could lead to the development of novel treatments that address the root cause of the disease. By understanding the mechanisms behind kidney failure in infantile cystinosis, researchers can now explore potential therapeutic options that may significantly improve the quality of life for patients with this rare condition.